
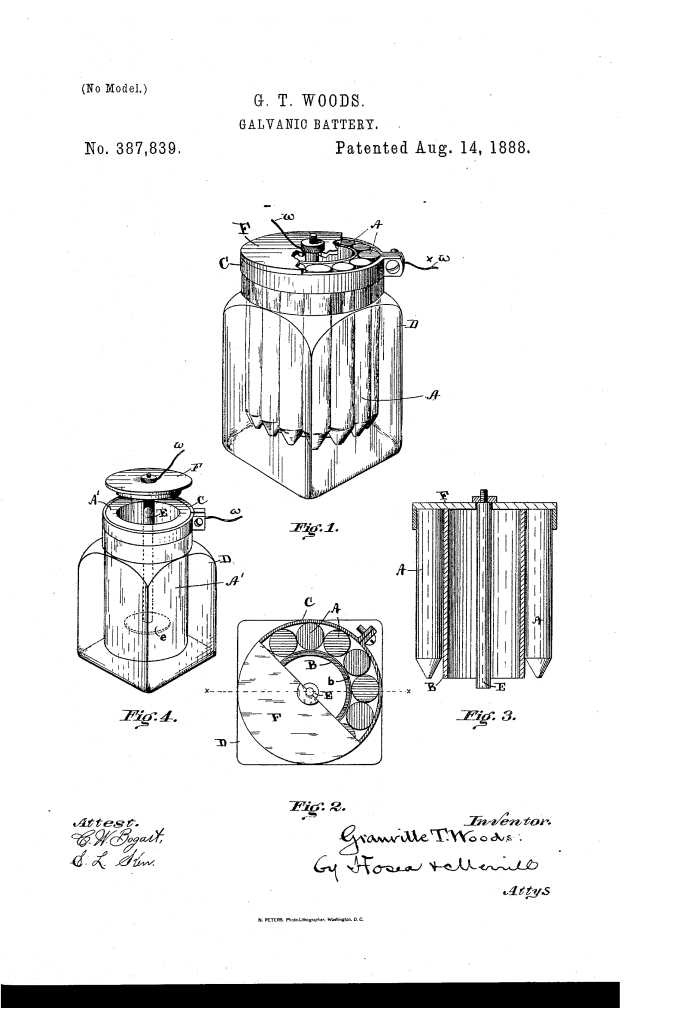
Galvanic Battery (Granville T. Woods, No. 387,839)
The patent by Granville T. Woods of Cincinnati, Ohio, describes an improved Galvanic Battery (Patent No. 387,839, 1888). This invention is a “wet cell” battery designed to be simple, inexpensive, and durable for the rapidly growing daily use of electrical apparatus in the late 19th century. Woods’s primary innovation was the introduction of an insulating shield between the electrodes, which forced a unique circulation of the exciting fluid. This design prevented “short-circuiting” at the surface and ensured the battery maintained a constant, long-lasting electrical output.
Inventor Background: Granville T. Woods
Granville T. Woods (1856–1910) was a prolific African American inventor and electrical engineer, often called “The Black Edison.” His work in the 1880s focused on the fundamental building blocks of the electrical age. While he is famous for the “Induction Telegraph,” his research into electrochemical cells was equally vital. During this era, batteries were the primary power source for telegraphs, telephones, and early electric bells. Woods applied his deep understanding of electrolytic action to solve the common problem of battery “polarization” and local short-circuiting, making power more reliable for the average consumer.
Key Mechanical & Chemical Systems
The battery is a “sal-ammoniac” style cell (similar to a modern Leclanché cell) housed in a glass jar.
1. The Electrode Assembly (A, E)
- The Negative Electrode (Carbon): A series of carbon pencils (A)—often the same type used in arc lamps—are arranged concentrically to form an open-ended cylinder.
- The Positive Electrode (Zinc): A central zinc rod or cylinder (E) is suspended from a wooden cover (F).
2. The Interposed Insulating Cylinder (B) (Key Innovation)
- The Shield: A thin shell of rubber or insulating material (B) is placed between the zinc and the carbon.
- The Path of Action: Because this cylinder is non-conductive, electricity cannot flow in a direct horizontal line between the zinc and carbon.
- Function: It compels the exciting fluid to circulate downward and then back upward around the bottom of the cylinder. This forces the electrochemical reaction to occur along the entire length of the electrodes rather than just at the surface.
3. The Metallic Band-Clamp (C)
- Structural Support: The carbon pencils are secured around the insulating cylinder by a metallic band-clamp (C).
- Function: This clamp does more than just hold the pieces together; it forms a circular support that allows the entire carbon assembly to rest on the neck of the glass jar (D). This makes the battery “modular”—the carbon and zinc elements can be lifted out independently for cleaning or replacement without falling apart.
4. Surface Accumulation Prevention
- Anti-Shorting Logic: In standard batteries, “surface bridges” (debris or chemical buildup) often form at the top of the liquid, causing a local short-circuit that drains the battery.
- Solution: By forcing the reaction to happen deeper in the jar (around the bottom of cylinder B), Woods’s design ensures that the strongest chemical action occurs where the zinc is most deeply immersed, keeping the top of the liquid clear and the current steady.
Improvements Over Standard Galvanic Cells
| Feature | Standard 1880s Batteries | Woods’s Improved Battery |
| Durability | Zinc rods corroded unevenly; frequent shorting. | Interposed cylinder (B) ensures uniform corrosion and prevents shorts. |
| Maintenance | Messy to refill; elements often broke during cleaning. | Modular assembly (Clamp C) allows for easy removal and fluid refilling. |
| Efficiency | Current weakened quickly due to surface buildup. | Forced circulation keeps the solution strength uniform. |
| Cost | Required specialized carbon plates. | Designed to use common, cheap carbon arc-lamp pencils. |
Significance to Electrical Engineering
Granville T. Woods’s galvanic battery influenced the development of chemical power sources and fluid dynamics in electronics.
- Controlled Ion Flow: Woods’s use of a physical barrier to dictate the path of ions is a foundational principle in modern battery separators, which are used in everything from lead-acid car batteries to lithium-ion cells to prevent internal shorts.
- Modular Component Design: The idea of an “all-in-one” removable electrode structure is a precursor to the replaceable battery cartridges used in industrial equipment today.
- Electrochemical Uniformity: By recognizing that the “strength of solution” must be uniform, Woods anticipated the active thermal and chemical management systems used in modern high-capacity battery packs.
- Resourcefulness: His decision to use arc-lamp carbons (a common industrial byproduct of the time) shows an early mastery of sustainable engineering and cost-reduction.
